Reaction of Alkyl Halides With Silver Nitrate
Two common methods of elimination reactions are dehydrohalogenation of alkyl halides and dehydration of alcohols. The catalytic system also converts methyl 2-isocyanobenzoate and amines into quinazolin-4.

What Is The Mechanism Of The Reaction Of Alkyl Halide With Silver Nitrite Quora
The reaction is driven toward products by mass action due to the precipitation of the insoluble salt.

. For unsymmetrical products the more substituted alkenes those with fewer hydrogens attached to the CC tend to predominate see Zaitsevs rule. Since two alkyl halides can react in three different ways therefore a mixture of three alkanes instead of the desired alkane would be formed. When an alkyl halide is used the reaction is called a dehydrohalogenation.
Silver nitrate catalyzes a reaction of primary amines with methyl αα-disubstituted α-isocyanoacetates to provide 355-trisubstituted imidazolones in very good yields via Ag-catalyzed insertion of the isocyano group into the N-H bond of the amine followed by in situ lactamization. Sodium iodide is soluble in acetone and sodium chloride and sodium bromide are not. In the classic Finkelstein reaction an alkyl chloride or an alkyl bromide is converted to an alkyl iodide by treatment with a solution of sodium iodide in acetone.
For example Wurtz reaction between 1-bromopropane and 1-bromobutane gives a mixture of three alkanes ie. For preparation of alkanes containing odd number of carbon atoms a mixture of two alkyl halides has to be used.

Solved The Reaction Of A Compound With Silver Nitrate In Ethanol The 1 Answer Transtutors
What Is The Mechanism Of The Reaction Of Alkyl Halide With Silver Nitrite Quora
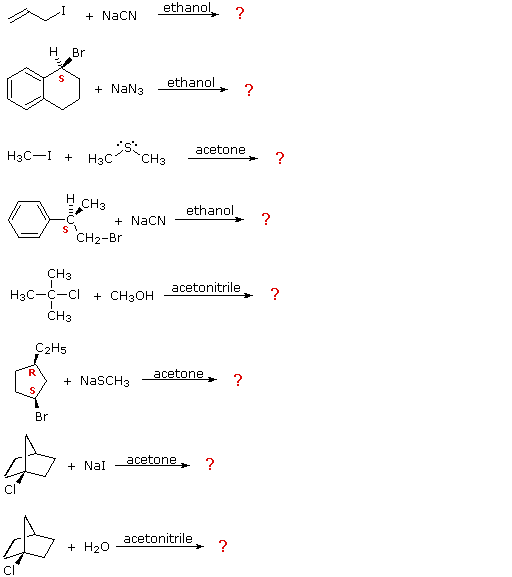
0 Response to "Reaction of Alkyl Halides With Silver Nitrate"
Post a Comment